What is Perchlorate?
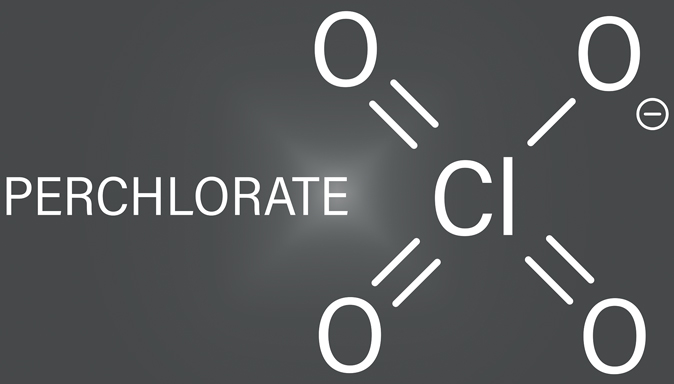
Perchlorate is a chemical compound comprising one chlorine atom and four oxygen atoms, represented by the formula ClO4-. It occurs both naturally and as an artificial substance. Naturally occurring perchlorate can be found in certain soils and mineral deposits, particularly in arid regions. However, most perchlorate found in the environment today results from human activities. It is widely used in various industries, particularly in producing rocket fuel, fireworks, explosives, flares, and some fertilizers. Because it’s highly stable and easily dissolvable in water, perchlorate can persist in soil and water sources for extended periods, potentially affecting water supplies.
Perchlorate can interfere with iodine uptake in the thyroid gland, which disrupts thyroid hormone production. These hormones are essential for metabolism and developing the central nervous system in fetuses and infants. For this reason, concerns have risen over the presence of perchlorate in drinking water, especially in areas near manufacturing facilities or military sites. Understanding how perchlorate contaminates water supplies, its effects on human health, and the best ways to remove it from drinking water is crucial for ensuring safe and healthy communities.
How Does Perchlorate Get Into Drinking Water?
The contamination of drinking water with perchlorate typically originates from industrial activities. When manufactured or used, perchlorate-based chemicals can leak into the surrounding soil and water supplies. In particular, facilities that produce or handle rocket fuel, fireworks, and explosives have a significant potential to contribute to local perchlorate contamination. Agricultural fertilizers can also introduce perchlorate into the environment, which may contain naturally occurring perchlorate from mineral deposits. When fertilizers containing perchlorate are applied to crops, runoff can carry the compound into nearby rivers, lakes, or groundwater sources, which are standard drinking water supplies.
Additionally, perchlorate contamination can occur when explosives or fireworks are used, as the compound disperses into the environment and can make its way into water supplies. Perchlorate’s stability and solubility in water make it particularly troublesome since it can travel long distances from the original contamination site. It does not quickly degrade, meaning it can persist in groundwater and surface water for years, ultimately making its way into the drinking water supply. In some instances, contaminated soil can leach perchlorate into groundwater supplies, adding another potential avenue for contamination.
Can Perchlorate Exist Naturally?
Yes, perchlorate can occur naturally, though this is less common than contamination from human-made sources. Natural perchlorate formation primarily occurs in extremely arid environments, where perchlorate can accumulate in soil and certain mineral deposits. Scientists believe natural perchlorate formation results from atmospheric reactions involving chlorine, ozone, and ultraviolet light. These reactions produce perchlorate, which deposits on the Earth’s surface, particularly in deserts. For example, large deposits of naturally occurring perchlorate have been found in Chile’s Atacama Desert, and small amounts have been detected in soils and groundwater across the southwestern United States.
Natural perchlorate has also been found in fertilizers derived from Chilean nitrate deposits. This leads to its incidental presence in agricultural products and water supplies when these fertilizers are used. Although natural sources contribute to the presence of perchlorate in the environment, human activities remain the primary cause of contamination in water supplies, especially in areas near industrial and military facilities.
What Does Perchlorate Do To Humans?
Perchlorate exposure is primarily harmful because it disrupts the thyroid gland’s ability to absorb iodine, which is a critical element for producing thyroid hormones. These hormones regulate various bodily functions, including metabolism, heart rate, and brain development in fetuses and infants. Because perchlorate interferes with iodine absorption, it can decrease thyroid hormone production. This can have a range of adverse effects, particularly for pregnant women, infants, and young children who are more sensitive to thyroid hormone disruption.
For pregnant women, exposure to perchlorate can pose a significant risk because thyroid hormones play a vital role in fetal development. Insufficient thyroid hormone levels in a mother can result in developmental issues for the baby, such as impaired cognitive function and delayed growth. Inadequate thyroid hormone levels can cause growth problems and cognitive deficits in children. For adults, exposure to high levels of perchlorate may lead to hypothyroidism, a condition characterized by fatigue, weight gain, and other metabolic issues.
The health risks associated with perchlorate have led regulatory agencies to assess its impact and establish guidelines for acceptable levels in drinking water. However, some scientists and health organizations continue to debate what levels should be considered safe, as even low-level exposure may have cumulative effects over time, especially in sensitive populations.
How To Remove Perchlorate From Water
Removing perchlorate from water can be challenging due to its stability and resistance to natural breakdown. However, there are several methods that water treatment facilities and even individuals can use to reduce or eliminate perchlorate from their drinking water.
- Ion Exchange – Water Treatment Plant: One of the most effective ways to remove perchlorate from drinking water is through ion exchange, a process commonly used in water treatment plants. In this method, water is passed through a resin bed that contains negatively charged ions. Perchlorate ions in the water are then exchanged with these negatively charged ions, effectively removing perchlorate from the water. While effective, ion exchange can be costly and requires regular maintenance to ensure continued effectiveness.
- Bioremediation – Water Treatment Plant: This method uses microorganisms to break down perchlorate into harmless byproducts, such as chloride and oxygen. In bioremediation, water is exposed to certain bacteria that can metabolize perchlorate as part of their energy cycle. Although bioremediation has shown promise, it is typically used in large-scale water treatment settings rather than individual homes due to the need for controlled conditions to support bacterial growth.
- Reverse Osmosis – Home: Reverse osmosis is a common method used in home water filtration systems to remove contaminants from drinking water. In this process, water is forced through a semi-permeable membrane that blocks perchlorate and other contaminants, allowing only clean water to pass through. Reverse Osmosis has a removal rate of 95%.
- Activated Carbon Filtration – Home: Activated carbon filters are less effective for removing perchlorate but can be useful when combined with other filtration methods. Activated carbon works by adsorbing specific contaminants onto its surface, though perchlorate is not as easily captured by carbon alone. As a result, activated carbon filters are generally used as a secondary treatment rather than a primary solution for perchlorate removal.
- Distillation: The most effective method of water purification is distillation, which involves boiling water and then condensing the steam back into liquid, leaving contaminants like perchlorate behind. Removal of perchlorate by distillation is over 99.9%.
Water Distillers Remove the Most Perchlorate
Perchlorate, a chlorine-based compound, is a contaminant of concern due to its widespread industrial use and persistence in the environment. It often enters drinking water supplies through industrial processes, agricultural runoff, and the use of explosives and fireworks. Once in the water supply, perchlorate can disrupt iodine absorption in the thyroid gland, leading to health risks, especially for vulnerable populations such as pregnant women and young children.
Understanding perchlorate’s health impacts has prompted the development of various water treatment solutions to remove it from drinking water. Distillation offers point-of-use water treatment that has proven to be the most effective method for removing perchlorate for those who wish to control their personal water quality. Given its potential health risks, continued efforts to monitor and mitigate perchlorate contamination are essential for protecting public health, especially as research advances in water purification technology.
Sources:
EPA: Perchlorate Fact Sheet (https://19january2017snapshot.epa.gov/sites/production/files/2014-03/documents/ffrrofactsheet_contaminant_perchlorate_january2014_final.pdf)
National Groundwater Association, “Perchlorate What It is and How to Remove It…”: https://www.youtube.com/watch?v=9uoYMpGPDeI
FDA: (https://www.fda.gov/food/environmental-contaminants-food/perchlorate-questions-and-answers#:~:text=What%20are%20the%20health%20risks,the%20production%20of%20thyroid%20hormone.)
University of Nebraska, “Perchlorate Toxicity and Risk Assessment” (Perchlorate Toxicity and Risk Assessment xicity and Risk Assessment)




We have used your water distillers (4) over the last 45 years to satisfaction. Drinking, ice cooking.
Thank you for this article.
How do you remove the perchlorate that your body absorbed before you started using a water distiller?
That would be a question for your trusted health care provider. We can only ensure that you won’t be introducing more of them once you start using the distiller.