What is an Electrolyte?
Every so often we come across people who claim the main reason for not wanting to drink distilled water is they have heard that distilled water does not have any electrolytes in it. This is really pretty much the same objection that people are giving when they say that distilled water does not have any minerals in it. In either case the person saying this is correct and we at Pure and Secure are proud of this fact…not ashamed. This is when one might ask, “Just what is an electrolyte anyway?”
 An electrolyte is any substance that forms ions (charged particles) when dissolved in suitable ionizing solvents such as water. (Positively charged ions are called cations and negatively charged ions are called anions.) Substances that ionize in water include most soluble salts, acids, and bases. There are a few gases that can function as an electrolyte under conditions of low pressure (or high temperature). A very good example of this is hydrogen chloride (HCl), a gas, which dissolves in water to form hydrochloric acid, a major component of gastric juices. Electrolyte solutions can also result from the dissolution of some biological compounds which contain charged functional groups. (Examples: DNA, polypeptides and some synthetic polymers)
An electrolyte is any substance that forms ions (charged particles) when dissolved in suitable ionizing solvents such as water. (Positively charged ions are called cations and negatively charged ions are called anions.) Substances that ionize in water include most soluble salts, acids, and bases. There are a few gases that can function as an electrolyte under conditions of low pressure (or high temperature). A very good example of this is hydrogen chloride (HCl), a gas, which dissolves in water to form hydrochloric acid, a major component of gastric juices. Electrolyte solutions can also result from the dissolution of some biological compounds which contain charged functional groups. (Examples: DNA, polypeptides and some synthetic polymers)
An example of how an electrolyte solution is formed would be when common table salt (sodium chloride) is added to water. The individual components of the salt dissociate (break apart) due to the interactions between the solute (salt) and the solvent (water). Chemists refer to this process as solvation.
NaCl(s) → Na+ (aq) + Cl− (aq) (aq stands for aqueous or in solution)
Another good example of solvation is when carbon dioxide (a gas) reacts with water (the liquid solvent) to produce ions. This example explains why when carbon dioxide from the atmosphere, reacts with distilled water (or any water for that matter), it produces a very slightly acidic solution.
CO2 + 2 H2O à H30+ (hydronium ion) + HCO3 – (hydrogen carbonate ion) all in solution
What is the physiological importance of electrolytes?
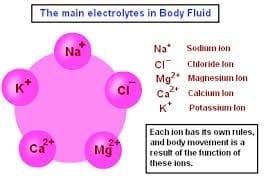
The primary ions (or electrolytes) of importance in human physiology are the following: sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), and chloride (Cl−). The electric charge symbols of plus (+) and minus (−) indicate that the substance is ionic in nature and has an imbalanced distribution of electrons which is the result of chemical disassociation. Sodium is the main electrolyte found in the extracellular fluid (watery solution) and is involved in fluid balance and blood pressure control. There are subtle and complex electrolyte balances throughout our bodies that affect several life functions. For example, the ratio of potassium to sodium ions in the intracellular and extracellular environment of cells determines the state of hydration or dehydration of our cells and our bodies. In addition, such ratios (referred to as osmotic gradients) also affect and regulate blood pH as well as nerve and muscle functions.
Fortunately there are various automatic bodily mechanisms in higher life forms (including mankind of course) that keep the concentrations of different electrolytes under tight control. Both neurons (nerve cells) and muscle tissue are considered electric tissues inside our bodies. Neurons and muscles are both activated by electrolyte activity which occurs between the extracellular and intracellular fluids.
Electrolytes can enter or leave the cell through specialized protein structures which are embedded in what is called ion channels in our plasma membranes. Without sufficient levels of these key electrolytes such as sodium, potassium and calcium, severe muscle contractions or muscle weakness may occur! Electrolyte balance is maintained by oral, or in emergencies, intravenous (IV) intake of electrolyte-containing substances, and is regulated by hormones, in general with the kidneys flushing out excess levels. Oral rehydration therapy may be necessary to replenish the body’s water and electrolyte levels after excessive exercise or excessive alcohol consumption. Electrolyte drinks containing sodium and potassium may be necessary in these situations. Other times when this therapy may be necessary is after bouts with diarrhea or vomiting.
This brings us back to the original concern. Do we depend on the water we drink for electrolytes and the answer is NO. No more than we depend on minerals in water for their other vital functions in our bodies. We depend mostly of our food, especially raw fruits and vegetables, for these very important nutrients…just as animals in nature do. If we do not feel that we are getting enough of these nutrients in our diets, we can take vitamin or mineral supplements (just make sure they are chelated or of the organic form). Then we can enjoy drinking pure, distilled water that will better help to deliver electrolytes (and minerals) to the proper locations in our bodies.




In much the same way cereal companies started the myth breakfast was the most important meal of the day, the 8 glasses of water a day was simply a “made-up” amount. There is no data to back that up. The human body needs more or less water each day depending on ones activities each day. Obviously there is a minimum. I have owned a Pure Water system for 3 decades and at 73 I am retired custom car builder and still building cars, but now for me, working on guns for me (I am a retired Police Dept. gunsmith) and I am designing a home for my wife and I. Did distilled water keep me healthy all these years? Who knows? It tastes better than the water from our supplier and building cars from the frame up, I have plenty of uses. With backup 12 volt power for home/farm lighting, my car and backup power batteries last much longer. Radiators don’t have scale either. It is not just for drinking. Use it in batteries and your windshield washer (no water spots). I wipe down show cars with it for the same reason. Thanks for listening.
Thank you for the ideas Charles. Distilled water has numerous uses and we are always finding more. Thank you for the response!
Sounds great! How did you compensate for the lack of minearals in the distilled water. Just about to purchase a distiller
I can’t speak for Charles, but most of the minerals in water are inorganic and our bodies are not absorbing anyways. Organic minerals come from a healthy diet. We get them from our food. Water is going to provide very little in this regard. If you do feel you are not getting the correct daily minerals in your diet, consult with a doctor or nutritionist and they would be able to direct you to some mineral supplements to add back to your water or to a change in diet. All you need from the water is just the water.
Rate means at 30 minute intervals. Quantity is the body weight in pounds divided by 2 and called ounces. 80 ounces is the maximum.
My guess is that the “right rate and quantity” is exactly how much water our bodies tell us to drink. Indeed, I wonder how it is that others can tell us how much to drink or eat. We will always be on the right track when we listen to our bodies, but we may most likely need to slow down the doing and the mental gymnastics we all seem so fond of in order to hear that still, small voice within us.
Please help me understand what do you mean “Drinking distilled water at the right rate and quantity …” so what is the right rate and quantity? Thank you very much!
Dr. Carey Reams’ research showed that too much salt in the blood causes plaque in the arteries. Drinking water with “electrolyte” salt in it does nothing to alleviate the situation. Drinking distilled water at the right rate and quantity keeps the arteries clear.