As the final article in our series on the dangers of fluoridation, one of the most important aspects we want to address is the effectiveness of distillation in removing fluoride compounds from drinking water. In independent laboratory testing of our distillers, the data showed greater than 99.9%+ removal of fluoride (they never say 100% removal).
Anyone who understands the process of distillation can easily predict the excellent results of our premium water distillers. Here’s why…
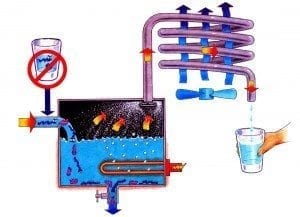 Distillation is the process of boiling water in order to turn liquid water into steam. When water is turned into steam it lets go of what it’s holding onto. Distillation then converts the pure steam back into liquid water, in a separate container. So in effect, instead of removing contaminants in water, distillation actually removes the pure water from the contaminants.
Distillation is the process of boiling water in order to turn liquid water into steam. When water is turned into steam it lets go of what it’s holding onto. Distillation then converts the pure steam back into liquid water, in a separate container. So in effect, instead of removing contaminants in water, distillation actually removes the pure water from the contaminants.
At standard temperature and pressure, (sea level), water (pure water that is) boils at 100° Celsius (212° Fahrenheit). Impurities in the water, whether organic or inorganic, elevate the boiling point and lower the freezing point from what it would otherwise be. If one of the impurities happens to be sodium or calcium fluoride, it would be left behind in the boiling chamber during distillation. This means that distilling raw water, softened or unsoftened, will remove the fluoride in any form.
The result: Our water distillers are extremely effective at removing fluoride (any form of fluoride) and they remain extremely effective for the life of the machine (which is a very long time). Our products are more effective than any filter or reverse osmosis system at removing fluoride. In fact, we can very confidently say that there is no other treatment process that is as effective and reliable at removing fluoride than distillation.
Read more about distillation and how to remove fluoride from water.



Thank you Crystle for your comment. I am not sure what you disagree with. While Mercola and Pure Water disagree on the best purification method for drinking water, we both agree that Fluoride is not needed in our drinking water and should be removed.
sodium fluoride is an ionic salt so yes it has a high boiling point. If you look at water quality reports from various municipalities however most of them are using fluorosilicic acid (non ionic), which has a boiling point of 105*C. Troubling for water distillery as a method of removing fluoride….
When hexafluorosilicic acid is heated, as it would be during the process of distillation, it decomposes into an ionic form. Therefore, it too remains behind as part of the residue water to be dumped as part of the maintenance.